Describe in Words the Meaning of Ka the Dissociation Constant
It is calculated by dividing the k off value by the k on value. This variation must be taken into account when making precise measurements of.

16 Determining Dissociation Constants
PKa - The pKa value is the negative base -10 logarithm of the acid dissociation constant Ka of a solution.

. K D is the dissociation constant and is the concentration of ligand which half the ligand binding sites on the protein are occupied in the system equilibrium. It is the concentration in Molar of analyte where 50 of the ligand is occupied by the analyte in a 1 to 1 interaction. Although the rate constants ka and kd are specific for a specific ligand-analyte pair they are independent of the concentration of both ligand and analyte.
Is pKa the same as pH. Dissociation constant Kd K the equilibrium constant involved in the dissociation of a compound into two or more compounds or ions. The dissociation constant or also known as kd is reverse of the freezing point depression constant also known as kf.
How to calculate your KD ratio. Dictionary Thesaurus Encyclopedia. Farlex Partner Medical Dictionary Farlex 2012.
In chemistry biochemistry and pharmacology a dissociation constant K D displaystyle K_D is a specific type of equilibrium constant that measures the propensity of a larger object to separate dissociate reversibly into smaller components as when a complex falls apart into its component. This equilibrium constant is a quantitative measure of the strength of an acid in a solution. The k denotes the word constant.
It is related to the acid dissociation constant K a by the simple relationship pK a pK b 14 where pK b and pK a are the negative logarithms of K b. Dissociation constant - the equilibrium constant for a reversible dissociation equilibrium constant - chemistry the ratio of concentrations when equilibrium is reached in a reversible reaction when the rate of the forward reaction equals the rate of the reverse reaction. By multiplying Ka by Kb you receive the Kw or the dissociation constant for water which is 1.
The dissociation constant of water is denoted K w. Dissociation constant noun Chemistry A quantity expressing the extent to which a particular substance in solution is dissociated into ions equal to the product of the concentrations of the respective ions divided by the concentration of the undissociated molecule. Redirected from dissociation constants Also found in.
There are tables of acid dissociation constants for easy reference. The reciprocal of the association constant 2. Ionization constant symbol K Learn More About dissociation constant.
How tightly a ligand binds to a particular proteinLigand-protein affinities are influenced by non-covalent intermolecular interactions between the two molecules such as hydrogen bonding electrostatic interactions hydrophobic. A constant that depends upon the equilibrium between the dissociated and undissociated forms of a chemical combination especially. Dissociation constant noun Chemistry A quantity expressing the extent to which a particular substance in solution is dissociated into ions equal to the product of the concentrations of the respective ions divided by the concentration of the undissociated molecule.
The base dissociation constant K b is a measure of basicitythe bases general strength. The dissociation constant is the ratio of dissociated ions products to original acid reactants. A rejection or denial of a belief or preconception.
Typically we determine the dissociation constant by seeing how much of. 2 The first second third etc. How do you calculate KD.
The dissociation constant is commonly used to describe the affinity between a ligand such as a drug and a protein ie. The concentration of water is omitted by convention which means that the value of K w differs from the value of K eq that would be computed using that concentration. For example H 2 S O 4 can lose one proton to make HSO4 which can then lose another proton to generate SO42.
KDA kills assists deaths for your kill-deathsassists ratio. The pH is a measure of the concentration of hydrogen ions in an aqueous solution. Need synonyms for dissociation.
The quantitative behavior of acids and bases in solution can be understood only if their pKa values are known. It is abbreviated as Ka. To learn more about Calculation of pka List of pKa values Relationship between pKa and pH and FAQs of pKa Visit BYJUS.
Dissociation constant Kd K the equilibrium constant involved in the dissociation of a compound into two or more compounds or ions. K a is commonly expressed in units of molL. Farlex Partner Medical Dictionary Farlex 2012.
The reciprocal of the association constant 2. The action of disconnecting or separating or the state of being disconnected. Heres a list of similar words from our thesaurus that you can use instead.
PKa acid dissociation constant and pH are related but pKa is more specific in that it helps you predict what a molecule will do at a specific. The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by K a. A breach of a harmonious relationship.
The equilibrium dissociation constant has a clear meaning. In this tutorial we look at the acid dissociation constant Ka and how it can tell us how dissociated an acid is in solution. Acid dissociation constants refer to the equilibrium constant for loss of the first second third and so on proton.
KdA constant that describes the relationship of the activities approximately concentrations of reactants and products in a chemical equilibrium of form A B AB. The dissociation constant Kd is the product of the activities of A and B divided by the activity of the product AB and has dimensions of concentration. The value of K w varies with temperature as shown in the table below.
In chemistry a base is a substance that can accept hydrogen ions protons or more generally donate a pair of valence electrons. In chemistry biochemistry and pharmacology a dissociation constant is a specific type of equilibrium constant that measures the propensity of a larger object to separate reversibly into smaller components as when a complex falls apart into its component molecules or when a salt splits up into its component ions.

Chapter 19 Acids Bases And Salts 19 3 Strengths Of Acids And Bases Ppt Video Online Download

At 25 Oc The Dissociation Constant Of A Base Boh Is 1 0 10 12 The Concentration Of Hydroxyl Ions In 0 01m Aqueous Solution Of The Base Would Be

Values Of The First Dissociation Constant Ka 1 Of H2s In Water At 25 C Download Scientific Diagram

Ka Association Constant Vs Kd Dissociation Constant Biochemistry Made Simple Youtube

Lesson Video Acid Dissociation Constants Nagwa
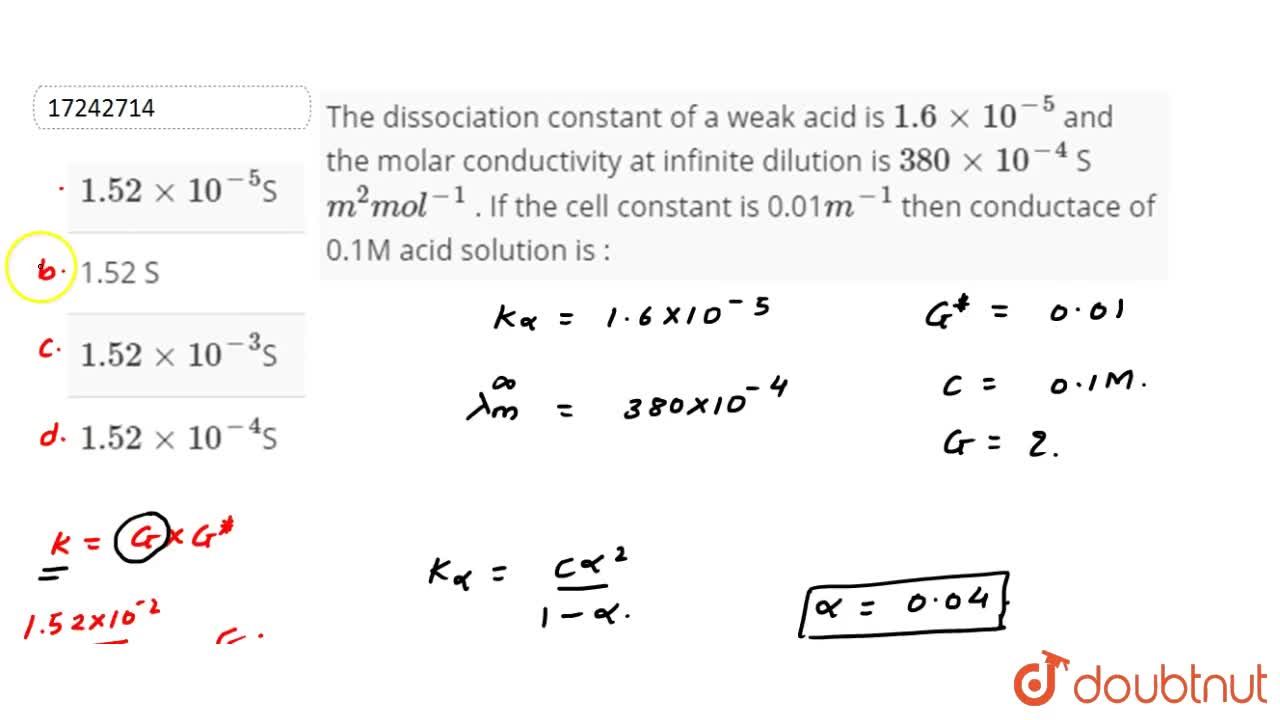
The Dissociation Constant Of A Weak Acid Is 1 6xx10 5 And The Molar Conductivity At Infinite Dilution Is 380xx10 4 Sm 2mol 1 If The Cell Constant Is 0 01m 1 Then Conductace Of 0 1m Acid Solution

The Dissociation Constant Of A Weak Acid Is 1 6xx10 5 And The Molar Conductivity At Infinite Dilution Is 380xx10 4 Sm 2mol 1 If The Cell Constant Is 0 01m 1 Then Conductace Of 0 1m Acid Solution

Values Of The First Dissociation Constant Ka 1 Of H2s In Water At 25 C Download Scientific Diagram

Spectrophotometric Determination Of The Dissociation Constant Of An Indicator Lab Report

What Is The Dissociation Constant Kd Fluidic Analytics

Pdf Determination Of The Dissociation Constant Of Some Substituted Phenols By Potentiometric Method In Acetonitrile Water Mixtures

Chapter 19 Acids Bases And Salts 19 3 Strengths Of Acids And Bases Ppt Video Online Download

Pdf Determination Of Acid Dissociation Constant Of Methyl Red By Multi Peaks Gaussian Fitting Method Based On Uv Visible Absorption Spectrum
What Is The Dissociation Constant Kd Fluidic Analytics

Chapter 19 Acids Bases And Salts 19 3 Strengths Of Acids And Bases Ppt Video Online Download

At 25 Oc The Dissociation Constant Of A Base Boh Is 1 0 10 12 The Concentration Of Hydroxyl Ions In 0 01m Aqueous Solution Of The Base Would Be
Why The Acid Dissociation Constant Ka Only Applies To Weak Acids Quora
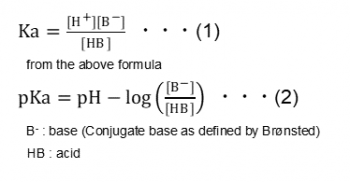
How Should The Acid Dissociation Constant Pka Be Measured Automatic Potentiometric Titrators Faq Kyoto Electronics Manufacturing Co Ltd Kem

Comments
Post a Comment